Health News
Aussie-invented NeedleCalmTM wins International Good Design Award

Health News
Time for action on a NSW Autism Strategy

Advertisements
Health News
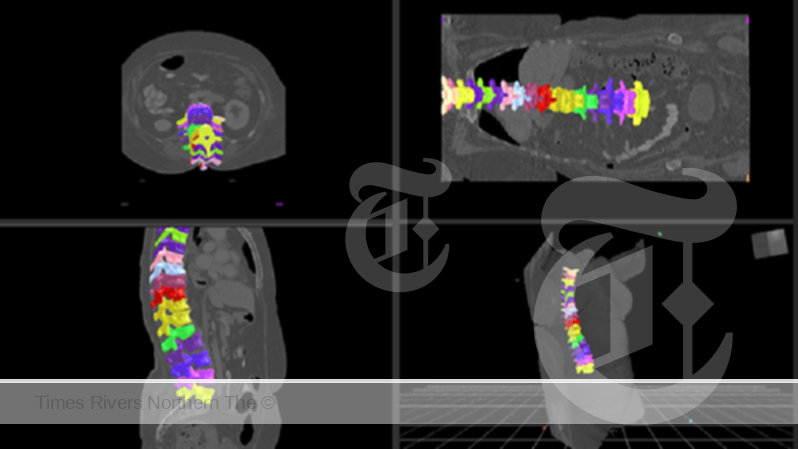
AI-Powered MedTech Breakthrough: CSIRO and Singular Health Unveil Revolutionary Spinal Vertebrae Segmentation Technology
Advertisements
Health News
Northern Rivers Business Community gets behind Autism Awareness Month

Advertisements
-

 Tweed Shire News2 years ago
Tweed Shire News2 years agoA NEW TWEED HEADS
-

 Motoring News1 year ago
Motoring News1 year agoToyota Supra: Get Ready For A Fully Electric Version In 2025
-
 COVID-19 Northern Rivers News3 years ago
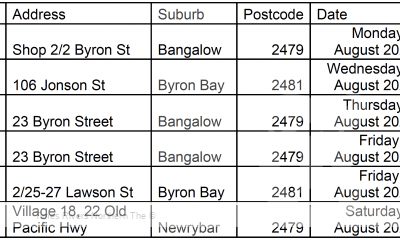
COVID-19 Northern Rivers News3 years agoNorthern Rivers Local Health District COVID-19 update
-

 COVID-19 Northern Rivers News3 years ago
COVID-19 Northern Rivers News3 years agoNorthern Rivers COVID-19 update
-

 Northern Rivers Local News2 years ago
Northern Rivers Local News2 years agoFears proposed residential tower will ‘obliterate’ Tweed neighbourhood’s amenity and charm
-
 Health News3 years ago
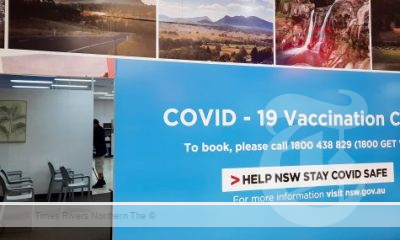
Health News3 years agoCOVID-19 Vaccination Clinic now open at Lismore Square
-

 COVID-19 Northern Rivers News3 years ago
COVID-19 Northern Rivers News3 years agoLismore Family Medical Practice employee close contact
-

 NSW Breaking News3 years ago
NSW Breaking News3 years agoVale: Former NSW prison boss Ron Woodham





























